Evolutionary Transition of Agriculture: CRISPR Technology
Dawn of agriculture
While humans had colonized all of the world’s continents by 13,000 years ago, the majority of them were still nomadic at the time, picking berries and hunting animals like traditional hunter-gatherer types. This changed around 11,000 BC, with the Neolithic Revolution, when a group of people established the Fertile Crescent. It descended upon an area that they saw was very suitable for the domestication of crops, and so they found wild species of grasses like Einkorn wheat, and actually started breeding them together to make them more suitable for producing food for humans. This wasn’t just limited to crops; they actually figured out the feasibility of domesticating large animals too, and they also invented complex systems of irrigation that brought human society together [1].
Across human civilizations, some of the first wild plants to be investigated were cereal grains. Grasses were domesticated into things like wheat, barley, maize, and sorghum. A really extreme example of this is teosinte, which is a tiny little grass that grows in parts of Mexico and was domesticated into modern corn over millennia [2]. Agriculture has really never been a natural process for as long as we’ve been growing foods as a part of our society. Instead, millennia after crop domestication, it has been an intentional process of plant modification. This was all happening through conventional breeding, where one plant was crossed with another, and their offspring would take some of the traits of their parents [3].
Development of Concepts of inheritance
In the 1850s, Charles Darwin’s Theory of Evolution revolutionized our understanding of inheritance’s true mechanics, which had been obscured since the early 1800s. Darwin was able to show that there was strong evidence for traits being passed down from parents to children in a predictable way. He also showed that this evolutionary process really did explain most of what had been seen in the domestication of crops and the evolution of wild organisms [4]. In 1866, Gregor Mendel, an Augustinian friar, greatly expanded this idea, known as Mendel’s Theory of Inheritance, by breeding peas together and observing the underlying trait segregation mechanism by which specific traits are inherited [5]. Despite his eloquent description of a gene, no one was able to understand what it was made up of. In the decades following his work, scientists sought to understand the material basis for the gene. This all came with the discovery and characterization of DNA in 1953 [6; 7]. The new-found understanding of DNA and genetic inheritance had huge implications for the way humans were able to improve crops.
A brief history of crop improvement
The Green Revolution (1950–1970), also called the Third Agricultural Revolution, led to a huge increase in crop yields and agricultural production [8; 9]. One key leader was agricultural scientist Norman Borlaug, the “Father of the Green Revolution,” who received the Nobel Peace Prize in 1970 for saving over a billion people from starvation. The main strategy was to develop high-yielding cereal grain varieties, build more irrigation systems, update management techniques, and spread hybrid seeds, synthetic fertilizers, and pesticides [10]. As crop improvement through selective breeding began to reach its limit, genetic modification technologies were developed to enable continued efforts [11].
Beyond enabling a huge transformation in agronomic productivity in the Green Revolution, the understanding of DNA inheritance also had huge implications for the tools that we could use to improve crops. One kind of quirky example of this is called an atomic garden. After World War II, American scientists and politicians were looking for new ways that they could use atomic energy for peacetime solutions. While the original purpose of these gardens was to observe the effects of radiation on plant life, scientists soon realized that radiation could be used to introduce beneficial mutations that would endow plants with desirable traits. Increased resistance to adverse weather is one example, as is a higher rate of growth. Atomic gardening is a mutation breeding technique in which plants are exposed to radiation [12]. Some of the resulting mutations have proven to be beneficial. Increased resistance to verticillium wilt of the peppermint is one example, as is a higher rate of growth [13].
Beyond random mutagenesis, scientists continued their journey to discover new molecular biological techniques for the insertion of genes into the genomes of organisms. This mutagenesis technique, like atomic gardening, and then also the insertion of foreign genes into plants, became pretty popular in the 2000s as a new means of producing diversity. The transgenic approaches evolved with developments in plant tissue culture. But still, the great feat of genome engineering through gene editing remained elusive, and that is to be able to very intentionally and specifically modify a gene within a very precise point within an organism’s DNA code. This all changed with the discovery of CRISPER/Cas9.
CRISPR/Cas9
Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) systems, an adaptive immune system in bacteria, have been modified for genome engineering. In the years prior to CRISPR, genome engineering approaches like zinc finger nucleases (ZFNs) and transcription-activator-like effector nucleases (TALENs) required scientists to create a new pair of nucleases for each genomic target. With its comparatively simple and adaptable mechanism, CRISPR is fast becoming the most popular method for genome engineering [14].
The development of the CRISPR-Cas9 genome editing technique was highlighted by the Nobel Prize in Chemistry in 2020, which was awarded to an American scientist, Jennifer A. Doudna, and a French scientist, Emmanuelle Charpentier [15; 16].
CRISPR/Cas systems have been categorized into two classes and five types, based on the classification of Cas protein. The type II CRISPR/SpCas9 system adopted from Streptococcus pyogenes has been modified and developed as versatile genetic engineering tools for different applications (Hsu et al., 2014), consisting of two key components: guide RNA, a type of RNA molecule that binds to Cas9 and specifies the location at which Cas9 will cut DNA, and Cas9, an enzyme that acts as “molecular scissors” to cut DNA at a location specified by a guide RNA [17; 18].
The technology allows researchers to perform the following: Gene Knock-out, DNA-free Gene Editing, Gene Insertions or “Knock ins” and Transient Gene [19; 20; 21]. Silencing. CRISPR/Cas9-based- gene-editing- technique in plant is comprised of various crucial steps as depicted in Figure 1, with selection of target sites, designing and synthesis of sgRNA, delivery of transformation carrier or ribonucleoprotein (RNP) in plant cells, transformation, and screening of gene-edited plants [22].

Figure 1. The procedure of CRISPR/Cas9-mediated gene editing in plants.
Source: [22]
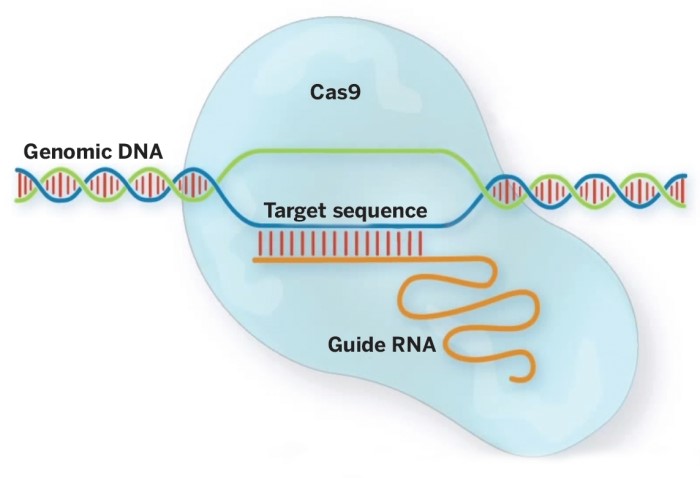
Figure 2. Cas9–gRNA complex cleaving genomic DNA Source: [23]
Here, in Table 1, is some research based on CRISPR/Cas9 for quality improvement of different crops.
| Crop | Associated Trait | Target Gene | References |
| Rice | Grain length and width | GW2, GW5, TGW6 | [24] |
| Rice | High amylose content | OsBEI and OsBEIIb | [25] |
| Rice | Low phytic acid content | OsPLDα1 | [26] |
| Tomato | Increased lycopene content | slyPDS | [27] |
| Tomato | Fruit size, oval fruit shape | OVATE, Fas, FW 2.2 | [28] |
| Tomato | Long shelf life | ALC | [29] |
| Wheat | Low gluten content | α-gliadin genes | [30] |
| Wheat | Grain shape | TaGW2 | [31] |
| Grape | Low tartaric acid | ldnDH | [32] |
| Orange | Resistance against cranker | CsLOB1 | [33] |
As in the wheat genome more than 100 loci are coded for gluten protein, a protein that could be responsible for triggering coeliac disease [34], conventional breeding methods can hardly reduce gluten content. In Contrast, the low-gluten, transgene-free wheat lines have been created with CRISPR/Cas9, targeting a conserved region of the α-gliadin genes [35]. Additionally, whole-genome sequencing results demonstrated that the incidence of off-target mutations caused by CRISPR/Cas9 in plants is relatively low. As per current USDA and FDA labeling standards, without GMO regulatory hurdles, CRISPR/Cas9 based crops are among non-GMOs, as this technique allows for precise genetic deletions or replacements for specialty crops [36; 37].
Conclusion:
Agriculture and humans have been transformed concomitantly, as a crucible of evolutionary change. CRISPR/Cas9 is a cutting-edge genome editing tool, with a wide range of real-world applications, which has versatile uses for improving desirable crop traits, such as crop quality, disease resistance, and many more. With precise genome engineering and transgene-free applications, CRISPR is expected to resolve the major challenges to crop improvement, opening new opportunities for agricultural endeavors to engineer plants’ traits.
References:
[1] Madden, R. (2021, July 8). The Development of Agriculture. National Geographic Society. Retrieved May 21, 2021, from https://education.nationalgeographic.org/resource/development-agriculture
[2] Cobb, J. (2022, May 19). domestication. National Geographic Society. Retrieved July 20, 2022, from https://education.nationalgeographic.org/resource/domestication
[3] Groover, E. (2020, June 23). CRISPR Crops: Food, Farms, and the Shape of Plants to Come. YouTube. Retrieved May 2, 2021, from https://www.youtube.com/watch?v=KKFoHXedP4g
[4] Kouprianov, A. (n.d.). Darwin, evolution, & natural selection (article). Khan Academy. Retrieved May 22, 2021, from https://www.khanacademy.org/science/ap-biology/natural-selection/natural-selection-ap/a/darwin-evolution-natural-selection
[5]Mendel’s principles of inheritance — Science Learning Hub. (2011, August 16). Science Learning Hub. Retrieved May 23, 2021, from https://www.sciencelearn.org.nz/resources/2000-mendel-s-principles-of-inheritance
[6] LunaDNA. (2019, April 24). History of DNA – What is DNA & How Was It Discovered? | LunaDNA. Luna DNA. Retrieved May 2, 2022, from https://www.lunadna.com/history-of-dna/
[7] The discovery of DNA – YourGenome. (2021, May 21). YourGenome. Retrieved May 22, 2021, from https://www.yourgenome.org/stories/the-discovery-of-dna/
[8] Eliazer Nelson, A. R. L., Ravichandran, K., & Antony, U. (2019). The impact of the Green Revolution on indigenous crops of India. Journal of Ethnic Foods, 6(1), 1-10.
[9] Ritchie, H. (2017, August 22). Yields vs. Land Use: How the Green Revolution enabled us to feed a growing population. Our World in Data. Retrieved May 31, 2021, from https://ourworldindata.org/yields-vs-land-use-how-has-the-world-produced-enough-food-for-a-growing-population
[10] Farmer, B. H. (1986). “Perspectives on the ‘Green Revolution’in South Asia”. Modern Asian Studies. 20 (1):
[11] Fukuda-Parr, S. (Ed.). (2007). The gene revolution: GM crops and unequal development. Earthscan.
[12] Ponsford, M., & Schlegel, E. (2021, May 27). A Short History of Atomic Gardening. NEO.LIFE. Retrieved May 29, 2021, from https://neo.life/2021/05/a-short-history-of-atomic-gardening/
[13] Van Harten, A. M. (1998). Mutation Breeding: Theory and Practical Applications. Cambridge, U.K.: Cambridge University Press. pp. 286–287. ISBN 978-0-521-47074-2.
[14] CRISPR Guide. (n.d.). Addgene. https://www.addgene.org/guides/crispr/
[15] Peltier, E. (2020, October 7). Two Scientists Win Nobel Prize in Chemistry for Crispr Gene Editing. The New York Times. https://www.nytimes.com/2020/10/07/science/nobel-prize-chemistry-crispr.html
[16] The Nobel Prize in Chemistry 2020 – Prize announcement. (2020, October 7). NobelPrize.org. https://www.nobelprize.org/prizes/chemistry/2020/prize-announcement/
[17] Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., & Charpentier, E. (2012). A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. science, 337(6096), 816-821.
[18] McDade, J. (2020, 3 26). Home. YouTube. Retrieved May 21, 2021, from https://blog.addgene.org/components-of-crispr/cas9-our-new-crispr-101-ebook?gclid=CjwKCAiA-8SdBhBGEiwAWdgtcGUydrqC19WOzkSsincGrTHpoMz9rxe81EBcKfh_nue_QRNuZviBohoCJAEQAvD_BwE
[19] Barrangou, R., Fremaux, C., Deveau, H., Richards, M., Boyaval, P., Moineau, S., … & Horvath, P. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science, 315(5819), 1709-1712.
[20] Dharmacon Edit-R CRISPR-Cas9 Gene Engineering System. (n.d.). Horizon Discovery. Retrieved May 23, 2021, from https://horizondiscovery.com/en/resources/featured-articles/dharmacon-editr-crispr-cas9-gene-engineering-system
[21] Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., & Charpentier, E. (2012). A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. science, 337(6096), 816-821.
[22] Liu, Q., Yang, F., Zhang, J., Liu, H., Rahman, S., Islam, S., … & She, M. (2021). Application of CRISPR/Cas9 in crop quality improvement. International Journal of Molecular Sciences, 22(8), 4206.
[23] Figure 2: Rainha, J., Rodrigues, J. L., & Rodrigues, L. R. (2020). CRISPR-Cas9: A powerful tool to efficiently engineer Saccharomyces cerevisiae. Life, 11(1), 13.
[24] Xu, R., Yang, Y., Qin, R., Li, H., Qiu, C., Li, L., … & Yang, J. (2016). Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. Journal of Genetics and Genomics= Yi chuan xue bao, 43(8), 529-532.
[25] Sun, Y., Jiao, G., Liu, Z., Zhang, X., Li, J., Guo, X., … & Xia, L. (2017). Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes. Frontiers in plant science, 8, 298.
[26] Lino, C. A., Harper, J. C., Carney, J. P., & Timlin, J. A. (2018). Delivering CRISPR: a review of the challenges and approaches. Drug delivery, 25(1), 1234-1257.
[27] Li, X., Wang, Y., Chen, S., Tian, H., Fu, D., Zhu, B., … & Zhu, H. (2018). Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Frontiers in plant science, 9, 559.
[28] Zsögön, A., Čermák, T., Naves, E. R., Notini, M. M., Edel, K. H., Weinl, S., … & Peres, L. E. P. (2018). De novo domestication of wild tomato using genome editing. Nature biotechnology, 36(12), 1211-1216.
[29] Yu, Q. H., Wang, B., Li, N., Tang, Y., Yang, S., Yang, T., … & Asmutola, P. (2017). CRISPR/Cas9-induced targeted mutagenesis and gene replacement to generate long-shelf life tomato lines. Scientific reports, 7(1), 1-9.
[30] Sánchez‐León, S., Gil‐Humanes, J., Ozuna, C. V., Giménez, M. J., Sousa, C., Voytas, D. F., & Barro, F. (2018). Low‐gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant biotechnology journal, 16(4), 902-910.
[31] Wang, W., Pan, Q., He, F., Akhunova, A., Chao, S., Trick, H., & Akhunov, E. (2018). Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. The CRISPR journal, 1(1), 65-74.
[32] Ren, C., Liu, X., Zhang, Z., Wang, Y., Duan, W., Li, S., & Liang, Z. (2016). CRISPR/Cas9-mediated efficient targeted mutagenesis in Chardonnay (Vitis vinifera L.). Scientific reports, 6(1), 1-9.
[33] Plant Breeding Innovation: CRISPR-Cas9. (n.d.). ISAAA. Retrieved May 19, 2021, from https://www.isaaa.org/resources/publications/pocketk/54/default.asp
[34] Hischenhuber, C., Crevel, R., Jarry, B., Mäki, M., Moneret‐Vautrin, D. A., Romano, A., … & Ward, R. (2006). Safe amounts of gluten for patients with wheat allergy or coeliac disease. Alimentary pharmacology & therapeutics, 23(5), 559-575.
[35] Sánchez‐León, S., Gil‐Humanes, J., Ozuna, C. V., Giménez, M. J., Sousa, C., Voytas, D. F., & Barro, F. (2018). Low‐gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant biotechnology journal, 16(4), 902-910.
[36] Globus, R., & Qimron, U. (2018). A technological and regulatory outlook on CRISPR crop editing. Journal of cellular biochemistry, 119(2), 1291-1298.
[37] Rappe, M. (2020, May 11). CRISPR Plants: New Non-GMO Method to Edit Plants. College of Agriculture and Life Sciences. Retrieved May 26, 2021, from https://cals.ncsu.edu/news/crispr-plants-new-non-gmo-method-to-edit-plants/

